
Pre-Launch Baseline
Proteus Digital Health
Pre-Launch Baseline
Proteus Digital Health
Proteus was a mature unicorn digital health startup with an FDA-approved cutting-edge ingestible ‘smart pill’ sensor for medication delivery, aimed at monitoring compliance. Ten years old and in growth mode they had raised $500M and had 200 people when I joined. Proteus filed bankruptcy in 2020.
This was a very innovative product that required patients to understand the value proposition and not be afraid of using it (it required swallowing a computer). This was the baseline study required to launch version 1.0 after product timeline slips, testing in parallel to release, pairing consumer research with the rigor of clinical trials. This research was conducted in an environment with regulated and unregulated products; hardware, software, and pharmaceutical components; and both physicians and patients as users.
This was a small, but rigorous study. This was the first time the company tested the complete working product before launch and the results gated the launch of the first release of the product.

Challenge
-
The product a completely new paradigm for patients to understand and want to use, so seeing the value of using it was key.
-
The schedule slipped three months. Four (4) rounds of formal testing planned, this was reduced to one (1).
-
The testing ended up occurring in parallel to the release.
-
Usability testing to-date was only able to evaluate static screens without real data over time.
-
Major innovative aspects of our product were being tested in use for the first time - over-encapsulation.

Product Release 1.0: medication (pills with a sensor), patient app, wearable patch, and instructions
What is the cumulative impact of our component-level usability issues?
Will users use our system over time?
Key Activities
-
Managed the research design and protocol development of this clinical study
-
Mentored and coached junior team members on research execution
-
Directed the analysis, synthesis, and data presentation.
-
Coached team in presenting findings to the executive team and broader organization.
Methodology
-
Pre-study:
-
The staff acted out the roles to test out the parts that we could test.
-
-
Study:
-
8 participants with chronic conditions (HEPC, CMB, HTN/Heart Failure)
-
The study was conducted over 9 days (2 patch changes)
-
Participants each attended 3 clinical visits, in person
-
IRB approved protocol
-
Rigorous clinical documentation
-

While we waited for a fully working system, the staff acted out the roles to test out the parts that we could test.
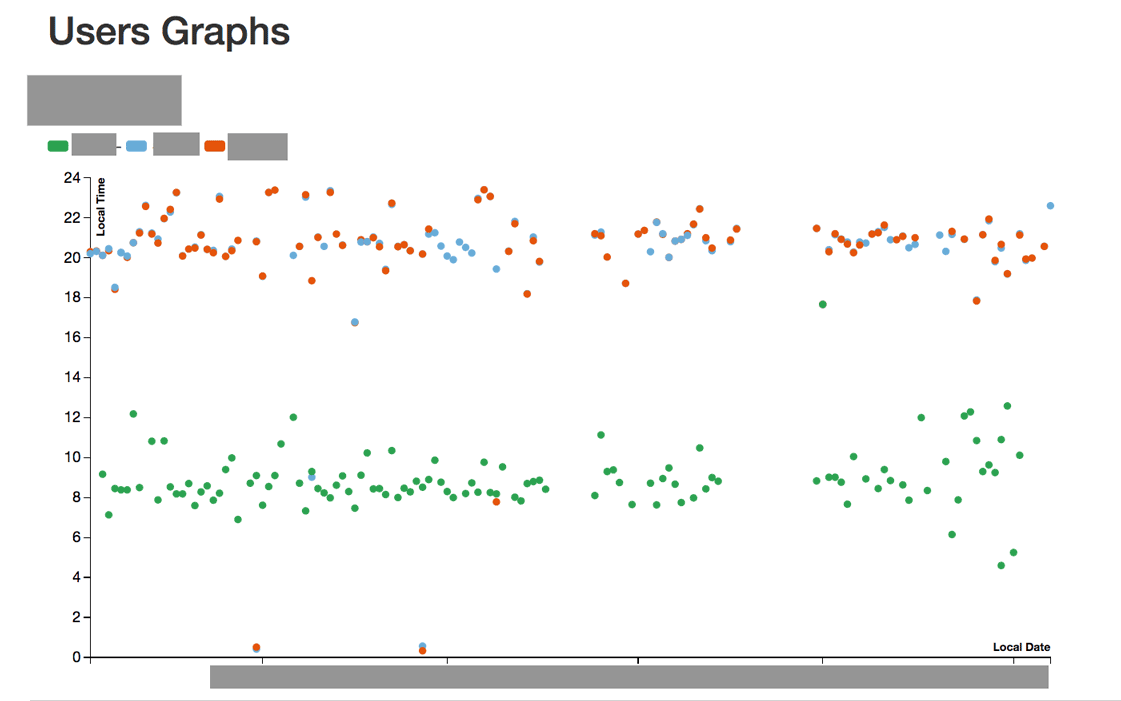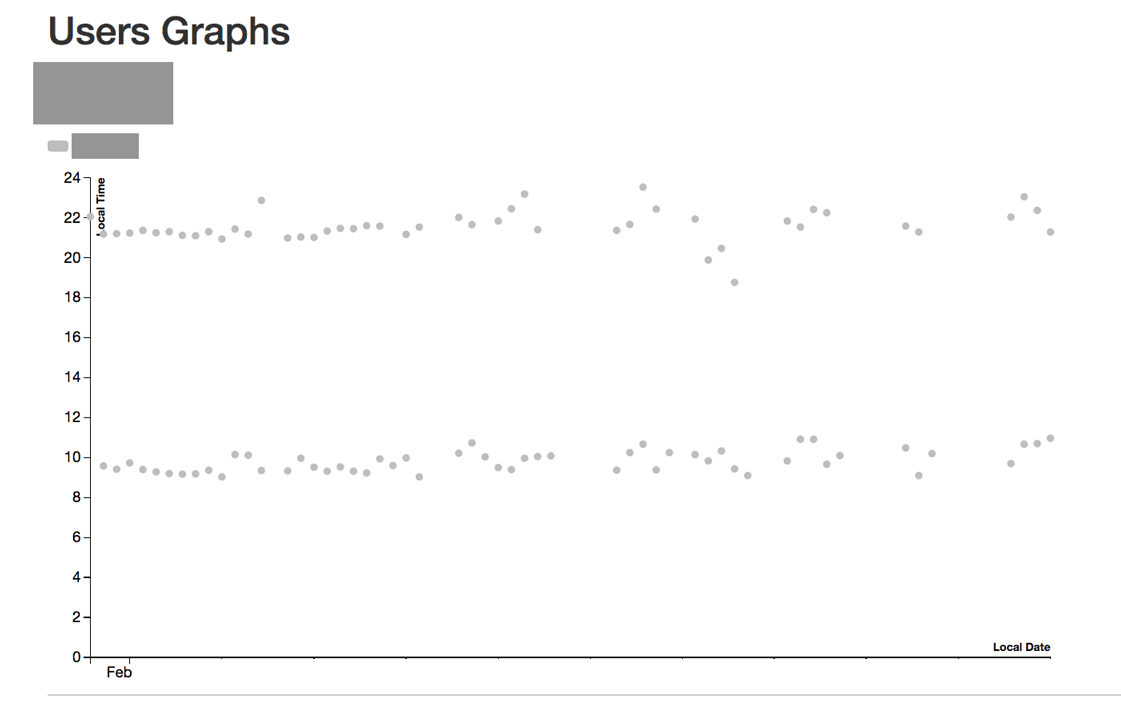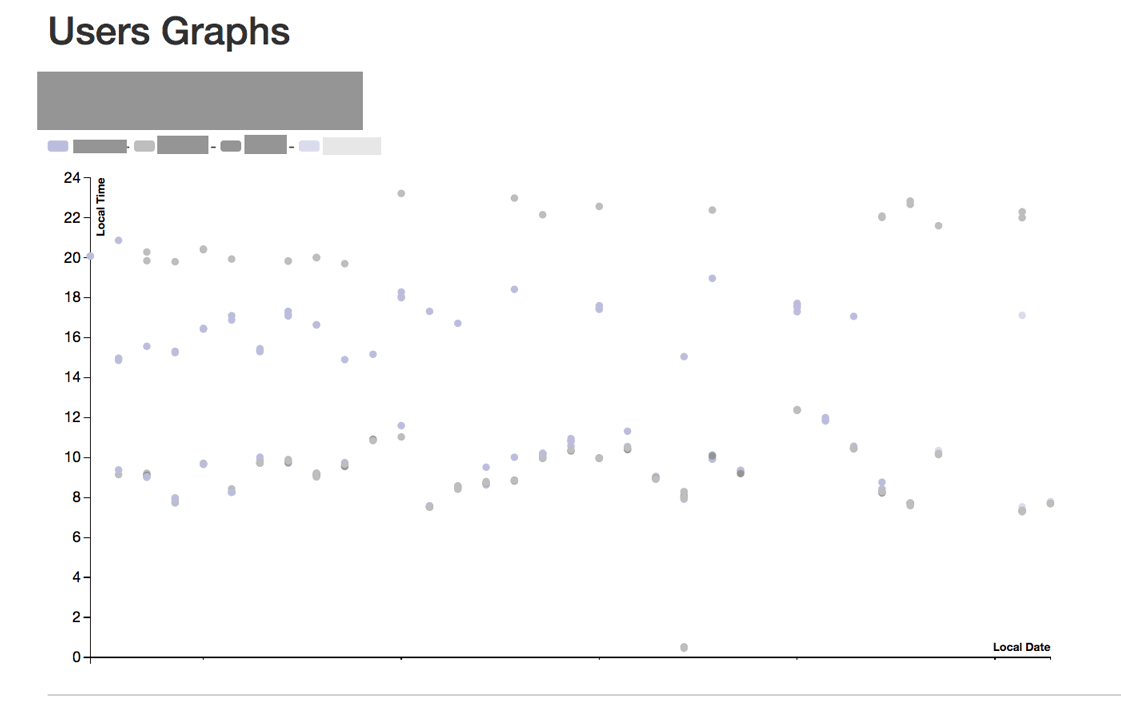
The participants pill-taking patterns (ingestion data) were integrated into the qualitative findings from the study to reveal real and perceived problems with data accuracy, latency and representation.
Image credit: Shruti Grover and Simon B Johnson (synthesized example of data shown)
Impact
-
Recommended to "launch and learn" from the first implementation. Due to timeline, we could not change the product, but we could change the instructions for training and onboarding patients.
-
The study insights revealed the wrong mental model (timeline, timestamps) which led to redesigning the 2.0 release into time buckets (morning, night), improved ease of use.
-
There was too much emphasis on tracking individual metrics so we changed training to highlight longitudinal patterns, clarified value prop
-
Determined the inaccuracy events erode trust (real or perceived) that led to improved algorithm requirements.

Innovations in research practice presented at the IDSA Medical Design Conference and Stanford's Medicine X
When Digital Meets Medical: The Next Generation Clinical Trial
Proteus Digital Health is pioneering a new product paradigm at the intersection of the medical and consumer worlds. We make regulated medical products that are used by regular people in everyday life, so they need to behave more like consumer products. User research, to refine medical products, is done using highly controlled clinical trials, while great consumer products are generally created using very different design research methodologies. Attendees will learn how cross-pollination of the clinical research with the best of consumer research opens up a whole new discipline ripe for exploration.
Published at IDSA Medical Design Conference and Stanford Medicine X.